
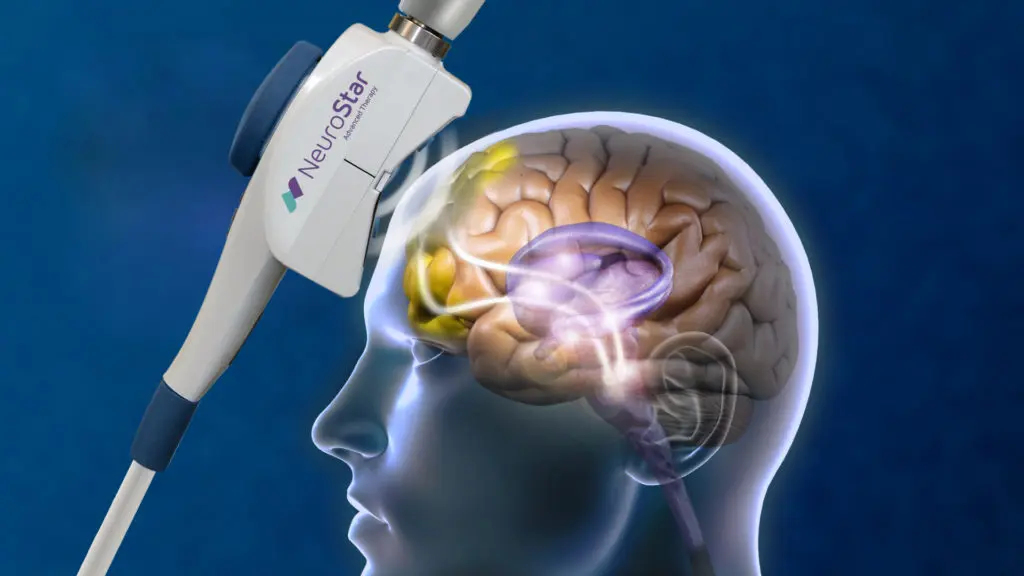
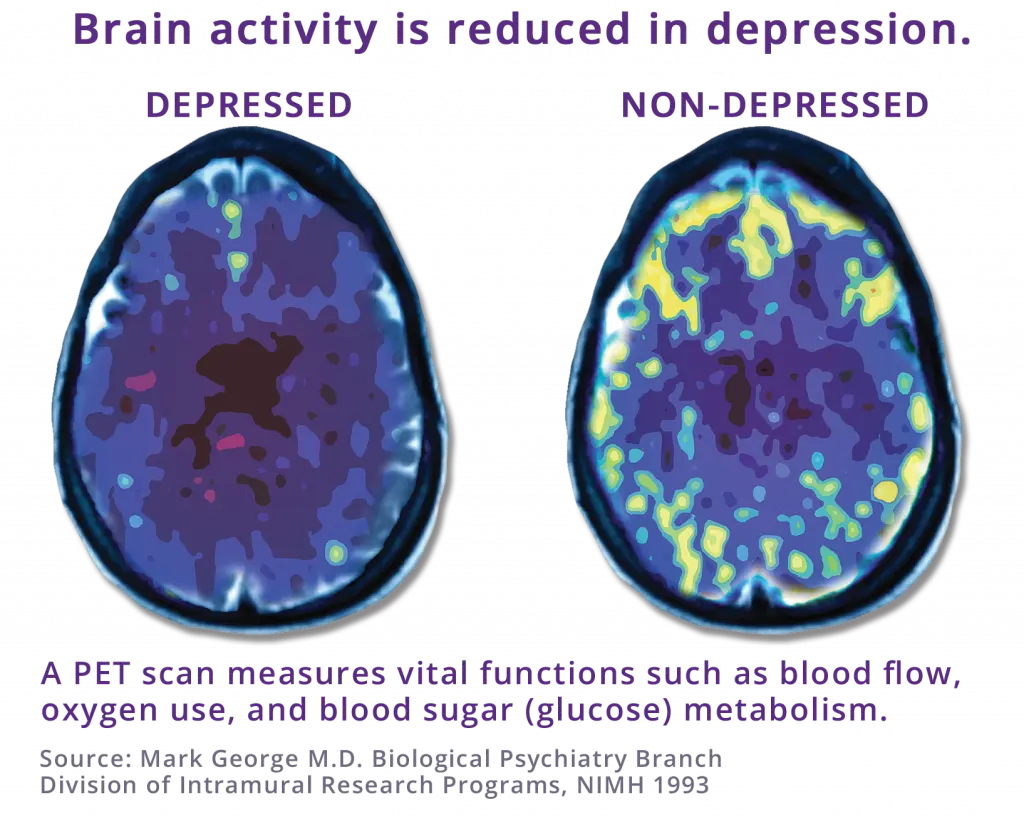
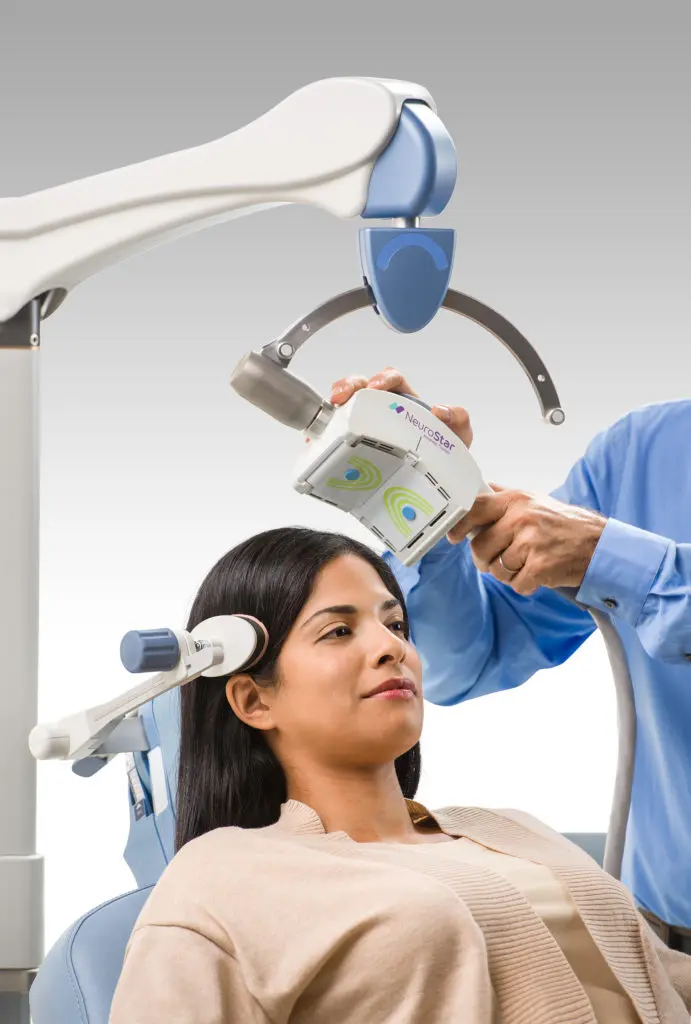
Transcranial magnetic stimulation, or otherwise referred to as TMS is a noninvasive procedure that uses magnetic fields to stimulate nerve cells in the brain to improve symptoms of depression. TMS is typically used when antidepressant medications haven’t been effective or have ceased working.
TMS involves delivering magnetic pulses to specific parts of the brain.
A typical initial course of treatment lasts about 19-37 minutes daily over a 4-6 week period.
A vast majority of commercial plans have recognized the effectiveness of depression treatment through TMS Therapy and now cover TMS as part of their plans.
TMS does not circulate in the blood throughout the body, so it does not have side effects like weight gain, dry mouth, sexual dysfunction, nausea, sedation, etc. The most common side effects that were reported during clinical trials were mild to moderate headache and scalp discomfort generally occurring less frequently after the first week of treatment.
No. TMS Therapy involves a unique method of using pulsed magnetic fields for a therapeutic benefit. The intensity of the magnetic field is similar to that of an MRI. These techniques differ from the popular use of low intensity, static magnetic fields. Those products deliver undirected and weak static fields that are incapable of activating brain cells. The activation and stimulation of brain cells is a key part of why TMS is so effective.